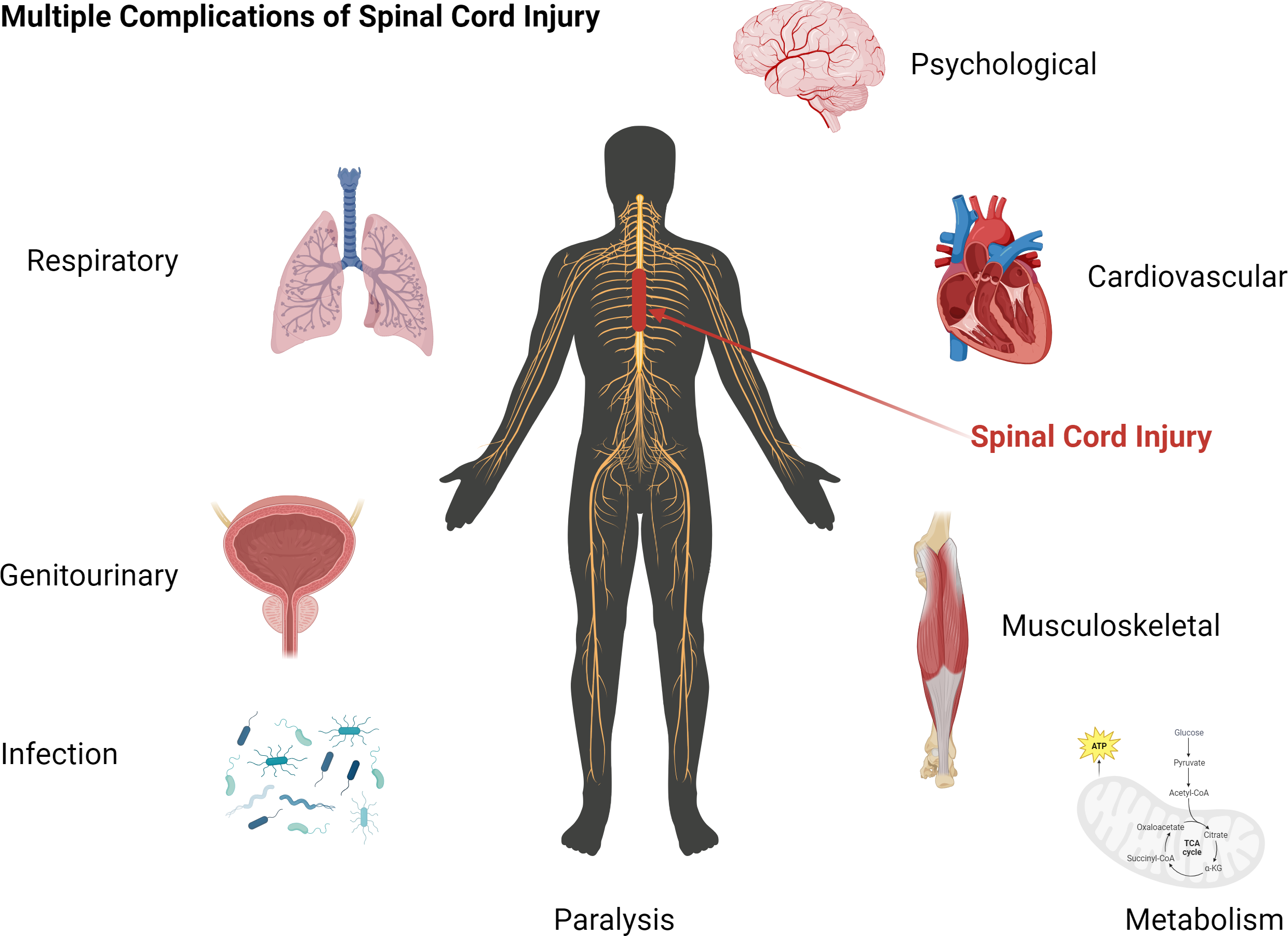
The physical impact/trauma that immediately damages the spinal cord tissue rapidly activates progressive degenerative events known as “secondary injury”. This secondary injury expands and, with time, affects the entire spinal cord, increasing the level of disability and diminishing the prospect of regaining neurological and functional recovery. Beyond the obvious paralysis that serve as an outward sign of the injury, SCI affects the whole body, including bladder, bowel, respiratory, cardiovascular, and sexual function — with social, psychological and economic implications. Neuropathic pain brings additional suffering to victims of SCI.
In the United States, more than 300,000 people live with SCI, with nearly 18,000 new cases added annually. Globally, more than 20 million individuals have long-term disabilities from SCI, and nearly a million more people suffer SCI each year. Young people with SCI face a lifetime of disability and personal and societal burdens, including a 2 to 5 times higher risk of premature death than the general population.
While the personal toll is profound, the economic toll of SCI is considerable. The annual healthcare cost for SCI patients in the US alone is approximately $60 billion. Despite the urgent need for treatments that preserve and heal the injured spinal cord, there are currently no approved treatments for SCI. Neupron is being developed as an early therapeutic intervention to protect the injured spinal cord from further degeneration and promote healing of the lesion cavity.
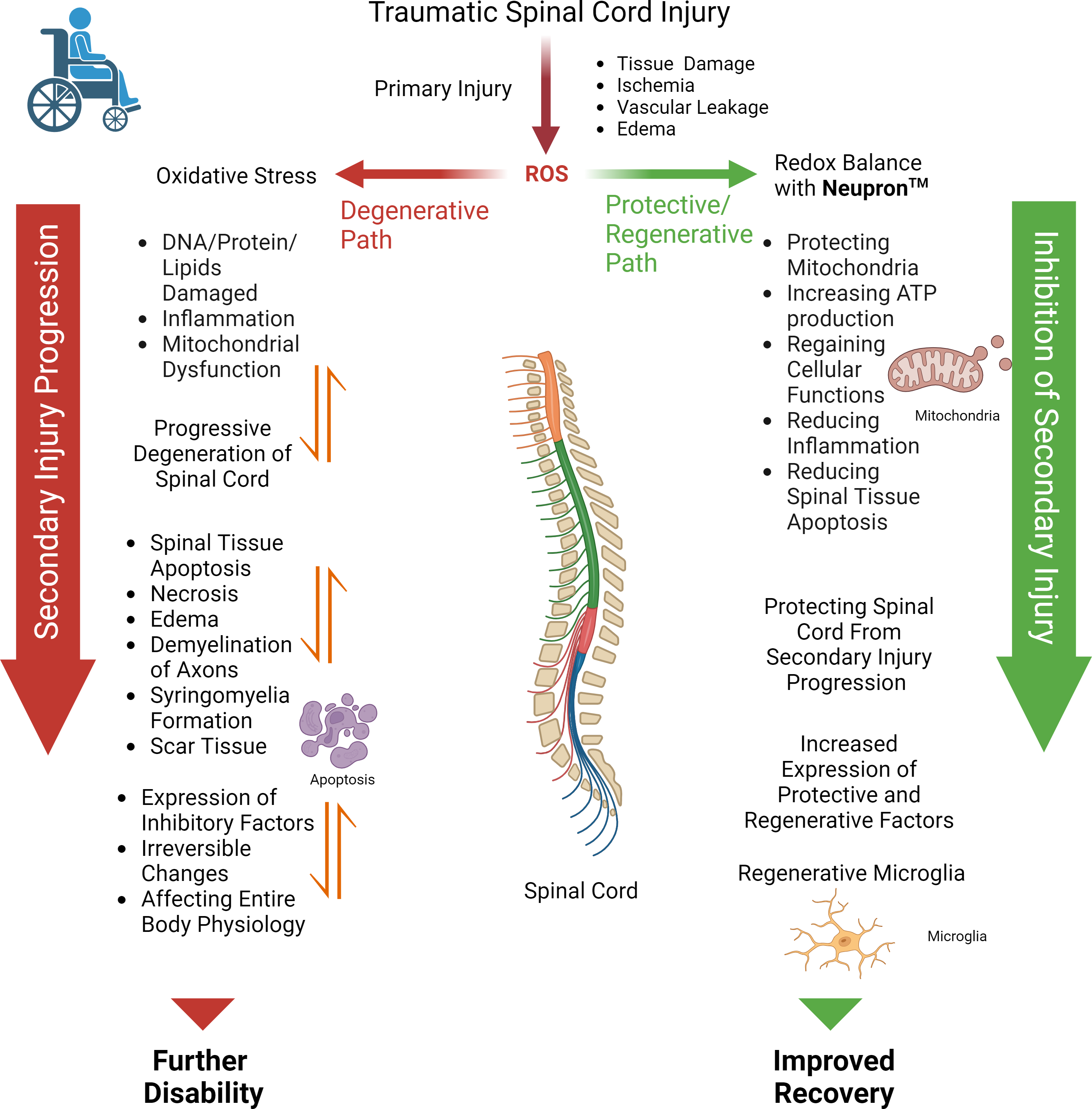
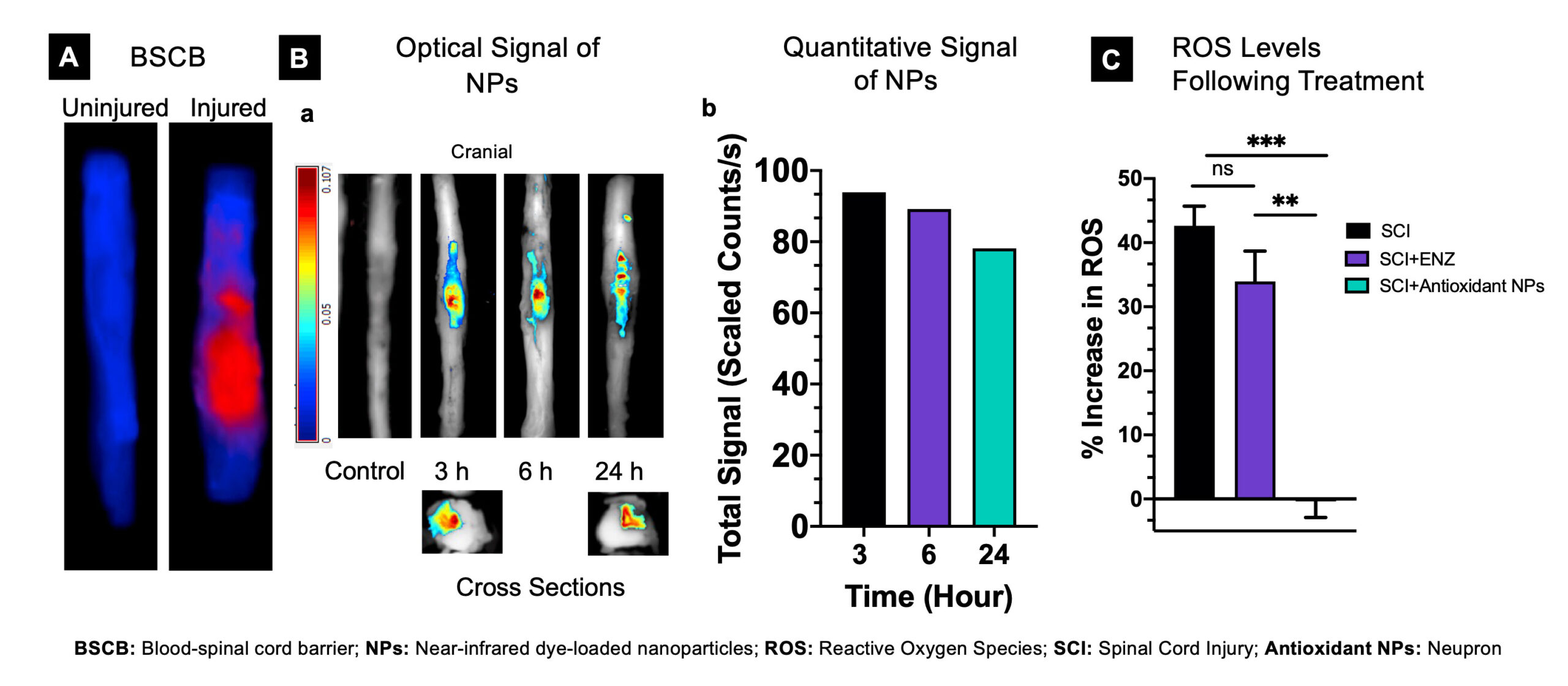
At AxoNeural Therapeutics, we want to change that. Soon after the SCI, there is excessive production of reactive oxygen species (ROS) at the lesion site, which acts as the key trigger to the cascade of degenerative events, causing inflammation and overexpression of apoptotic factors, resulting in further spinal tissue death. This self-propagating progressive degeneration eventually affects the entire spinal cord, exacerbating disability with time.
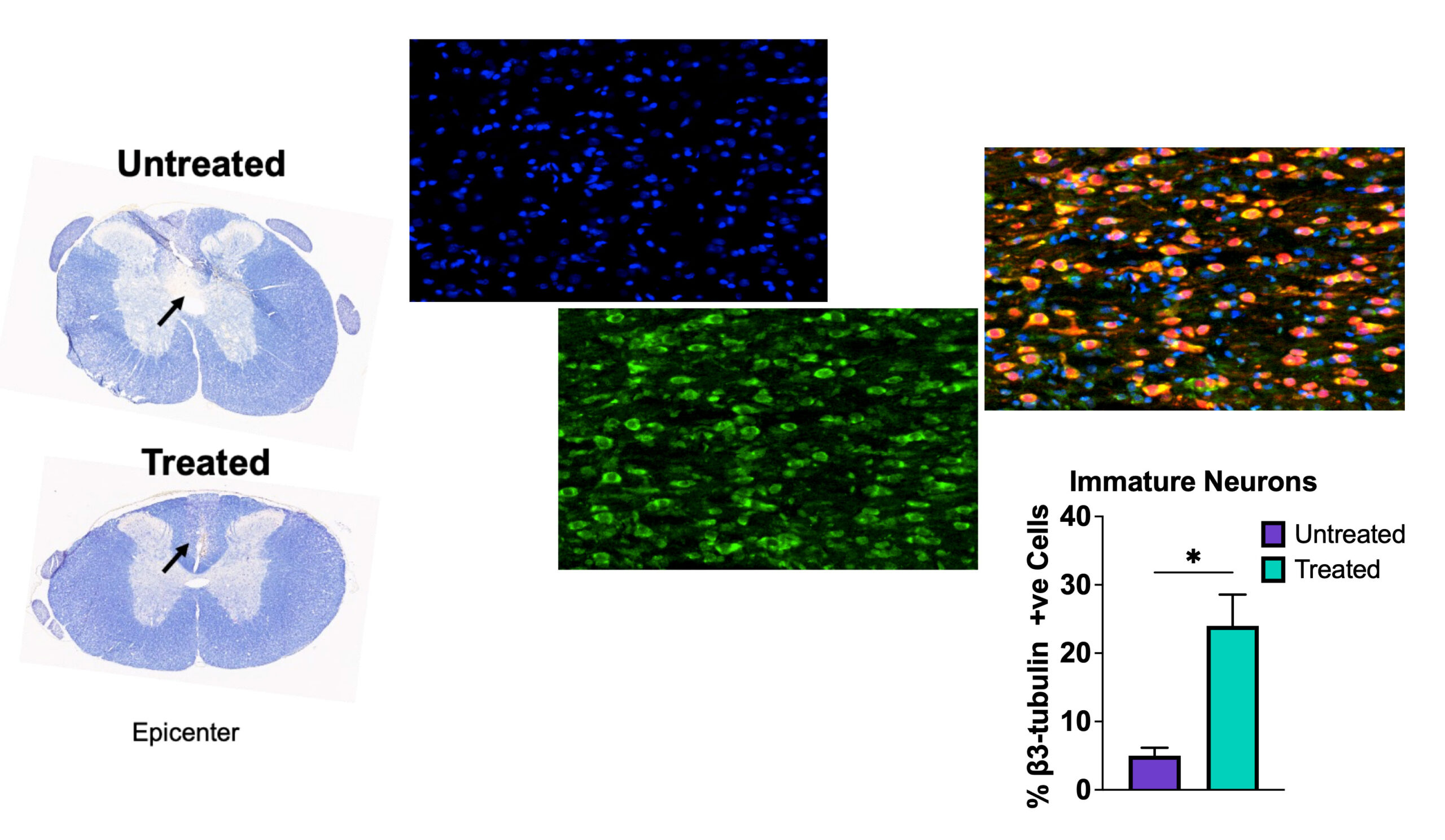
Neupron is a patented injectable formulation of nanoparticles encapsulating antioxidant enzymes. In a preclinical model study of SCI, intravenous injection of Neupron is shown to localize at the injured site, neutralizing the toxic effect of ROS. The regaining of redox balance at the lesion site is demonstrated to protect the injured spinal cord from progressive degeneration, promoting healing of the lesion cavity thus regaining recovery. AxoNeuronal Therapeutics intends to develop Neupron as an early therapeutic intervention to stabilize the injured spinal cord. This is anticipated to facilitate recovery from spinal cord injury to improve the quality of life.
Changing the dynamics in injured spinal cord: By restoring the redox balance at the lesion site, Neupron changes the dynamics in the injured spinal cord microenvironment from degenerative to regenerative, thus protecting and promoting healing.
Impact of SCI on vital organ functions: Injury to the spinal cord impacts nearly every organ in the body, and hence it is commonly referred to as “Multi-organ Disease”. Following SCI, increased systemic levels of inflammatory cytokines and reactive oxygen species, cause damage to vital organ functions.
While Neupron has its main effect at the site of spinal cord injury, its initial systemic distribution protects vital organs from damaging effect of reactive oxygen species.